Theory
The reaction speed of the brilliant green solvolysis is investigated as function of pH and the reaction speed constant is determined.
Brilliant green solvolysis
Kinetics
The dark green compound brilliant green (B) reacts with water or hydroxide ions to the carbinol base (C) which appears colorless (figure ). The reaction kinetics can be described using the reaction speed constants k_1 and k_2 as
Thus, the reaction kinetics are of second order. By using two approximations we simplify the kinetics to a first-order equation:
-
The concentration of water is constant.
This approximation is reasonable, since aqueous solutions are made up of water molecules and the concentration of water does not change significantly in the course of the reaction. The concentration of water is thus a constant that can be merged into a new reaction speed constant k'_2.
k'_2=k_2\,c_\ce{H_2O} -
The concentration of hydroxide ions is constant. This approximation is justified if the used solution is kept at constant pH using a buffering solution.
k'_1=k_1\,c_\ce{OH^{-}}
With these approximations, equation is simplified to
The decomposition of the reaction speed constant k as
allows to determine the quantities k'_2 and k'_1 by plotting the total reaction speed constant k against the concentration of hydroxide ions. Now, the reaction kinetics are of first order.
The equation can be solved by separation of the variables and subsequent integration.
The term c_\text B(t_0) describes the concentration of brilliant green at time t_0. It is useful to consider the time t_0 as the start of the measurement, thus t_0 = 0.
Lambert-Beer law
If monochromatic light with intensity I_0 travels through a light-absorbing substance, the intensity of the outgoing light I is reduced. The differential reduction of the intensity -\mathrm dI is in good approximation proportional to the intensity I within the differential travel distance \mathrm d\ell. The proportionality constant is called k'.
Using separation of variables and subsequent integration results in the Bouguer-Lamber law.
A new constant k is introduced to convert from the natural logarithm to the decadic logarithm. The decadic logarithm of the ratio \frac{I_0}{I} is called extinction E.
According to the Beer law the proportionality constant k is proportional to the concentration c.
Here, \varepsilon is the molar decadic extinction coefficient. Inserting equation in equation yields the Lambert-Beer law:
The following prerequisites are required for the Lambert-Beer law to be valid:
-
The Bouguer-Lambert law only applies for monochromatic light in a optically homogeneous medium.
-
The Beer law only applies for solutions in which no concentration-dependent equilibrium forms.
-
The Lambert-Beer law applies for solutions with low concentrations, as interactions between the molecules are not considered.
Isosbestic point
A simple method allows to check whether the concentration of reaction educts and products is described using a fixed stoichiometric ratio. This allows to confirm whether the reaction takes places without additional follow-up or side reactions . The extinction can be measured as a function of wavelength, resulting in a spectrum. In case of a reaction, in which two absorbing compounds A and B take part, the extinction is
The molar extinction coefficients are wavelength-dependent properties. Their ratio can be described using a factor \nu.
Using this relationship in equation yields:
We now focus on the case that \nu is the stoichiometric ratio, since the term in parenthesis is the total concentration c_0. Then the right-hand side of the equation consists only of constants.
This means, that the extinction is constant for all wavelengths at which the ratio of the molar extinction coefficients equals the stoichiometric ratio. These points are called isosbestic points. If multiple spectra taken at different times during a reaction exhibit points at which the extinction is constant at one or more particular wavelengths, these points are called isosbestic points. The number of isosbestic points that can be observed depends, among others, on the spectrum of educt and product compounds.
Extinction measurements of brilliant green solutions
Brilliant green absorbs light in the visible spectrum and appears green. In contrast, the carbinol base appears colorless. The reduction of brilliant green concentration as a function of time can thus be followed using a spectral photometer . Since the molar extinction coefficient of brilliant green \varepsilon is rather larger, even small amounts of the substance result in greenly-colored solutions. Using the Lambert-Beer law (equation )
in equation yields with t_0 = 0 the equation
Here, E_0 is the extinction at time t = 0 (i.e. at the start of the measurement). This relationship allows to determine the reaction speed constant be plotting \ln\left(\frac{E}{E_0}\right) as function of the measuring time t and determining the slope using a linear fit. It is important to note that equation only holds if the extinction E is measured at a wavelength at which only brilliant green absorbs light. In order to check for this prerequisite, the individual spectra of the pure substances brilliant green and the carbinol base have to be recorded. While this measurement is straight forward for the case of brilliant green, the spectrum of the carbinol base can be only obtained indirectly, as it is produced during the reaction. For this reason, multiple spectra are recorded at different time offsets.
pH value
The pH of a solution is the negative decadic logarithm of the concentration of oxonium ions. Per definition, the concentration of oxonium ions is expressed in units of mol / L.
The pH can be determined using a glass electrode. Analogously, the pOH value is defined as
From the ionic product of the autoprotolysis reaction of water follows that
Experimental setup
In this lab course the kinetics of the brilliant green solvolysis is investigated as function of pH at constant temperature. The pH is measured with a digital pH meter with a glass electrode. The concentration change in the solution is determined by measuring the absorption using a spectral photometer with a temperature-controlled sample holder.
Spectral photometer
A commercial spectral photometer is used. The setup is schematically shown in figure .
The purpose of the light source is to provide a broad spectrum of light. Typically, tungstic, deuterium or xenon lamps are used (the latter is used in the lab course). The monochromator (a prism or diffraction grating) splits the incoming light according to the wave length, such that light from a narrow part of the spectrum reaches the sample. In this lab course, a diffraction grating is used. After passing through the sample, the light intensity is converted into an electric signal by using photo multipliers or photo diodes.
The optical beam bath is shown in figure .
The used cuvette is made from fused silica, since normal glass would absorb light in the used wavelength range. To perform the measurement at a fixed, controlled temperature, the sample holder is placed in a temperature-controlled surrounding. A thermostat is used to adjust the temperature.
The cuvette has two matt and two optically clear sides for passing through the beam. Make sure that the clear sides are used for the light beam and do not touch these sides with your hand. Use the matt sides to hold the cuvette with your hands.
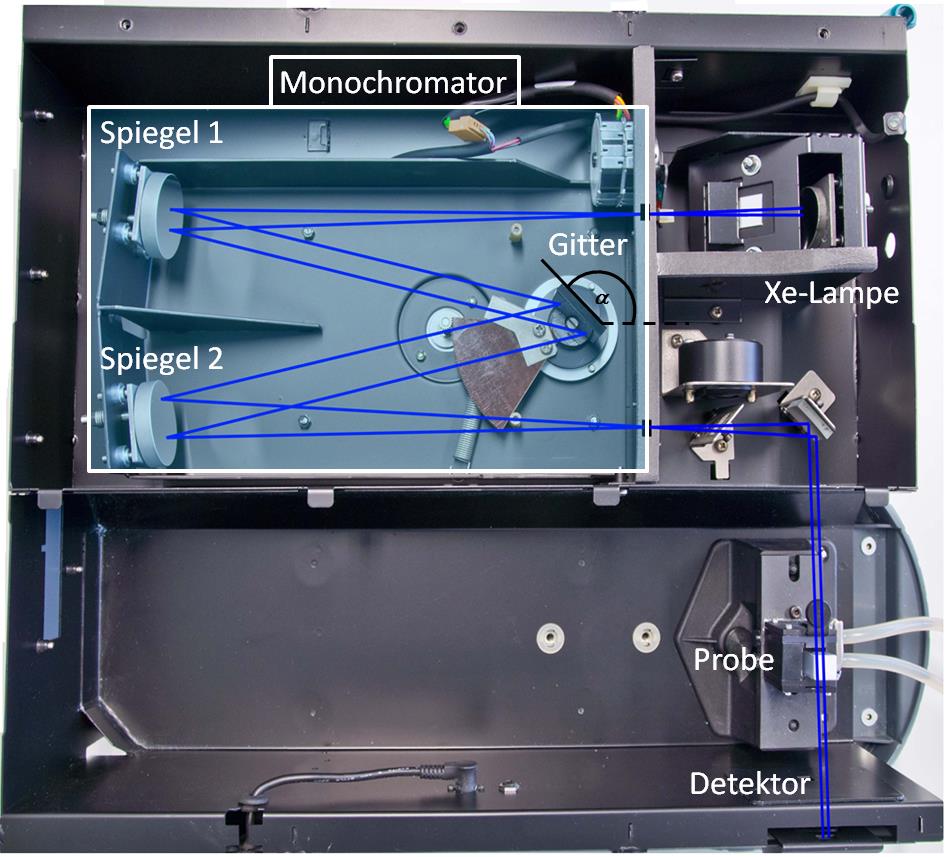
Spiegelmeans mirror,
Gittermeans grating,
Lampemeans lamp,
Probemeans sample).
Chemicals
The following stock solutions are provided:
-
Aqueous brilliant green solution with a concentration of 0.000104 mol / L.
-
Four buffer solutions A, B, C and D consisting of an aqueous Borax stock solution (disodium tetraborate \ce{Na_2B_4O_7}, 0.025 mol / L). The pH of the solutions was adjusted using aqueous potassium hydroxide solution (0.1 mol / L). Since different ionic strengths would influence the measurement, an aqueous potassium chloride solution (0.1 mol / L) was used to keep the ionic strength of all solutions constant.
Instructions
Virtual lab course video tutorial
Go to virtual lab course (Part 1, recording spectra) Go to virtual lab course (Part 2, time-dependent measurements)Lab course instructions
Preparation
-
Turn on the computer and login with user .\Praktikum and password Passwort*.
-
Set the thermostat to a temperature of 25 °C and turn on the cooling circulation.
-
Measure the pH of the buffer solutions using the pH meter at any point in time during your time in the lab.
Notes for the interactive experiment Wait about 10 seconds until the pH value has reached a constant value after selecting the solution. Then click on Restart reaction and then on Record new spectrum or Record new time series.
Extinction spectrum of brilliant green
Go to virtual lab course (Part 1, recording spectra)Record an extinction spectrum of brilliant green at 25 °C in a wavelength range between 250 nm to 800 nm.
-
Insert the cuvette into the tempering housing of the photometer and add a volume of 2.5 mL of water with the micro pipette to the cuvette.
-
The Celsius temperature of the cuvette with the sample is automatically measured and displayed by the spectrophotometer. Wait until this has a constant Celsius temperature of 25 °C for a period of three minutes.
-
Start the software Spektrum Brillantgrün. The software indicates that no baseline correction is present. Confirm both alerts with Ok.
-
Create a baseline correction by pressing Baseline. The process is finished if the program is responsive again.
-
Measure now the spectrum of brilliant green. Press Start and choose a filename for your measurement. Select the folder D:\Userdaten\, create a subfolder with your group number and save your data as a CSV file. Confirm with Save.
-
Add 0.3 mL of brilliant green solution using the micro pipette to the water in the cuvette. Carefully mix the solution with the micro pipette (by inserting and releasing liquid into the micro pipette).
-
In the field Sample Name press Ok to start the measurement (the name given here does not play a role). A window with a two-minute timer will then appear. During this time, press Ok again to start the measurement.
-
Follow the measurement on the monitor. Meanwhile, the programme creates five graphs one after the other. The measurement is finished as soon as Start is highlighted in light grey again.
-
When the measurement is finished, press Finish in the window Sample Name.
-
Determine the wavelength of the extinction maximum from the diagram and make a note of it. For a closer look at the extinction maximum, it can be enlarged by holding down the left mouse button and framing a section of the diagram. Close the program. Your data is automatically saved.
-
Remove the cuvette from the sample holder and check the cuvette for impurities or air bubbles. Empty the cuvette into the waste container, clean the cuvette with pure water and dry the cuvette with lintfree cloth.
Notes for the interactive experiment The baseline correction is unnecessary. Use buffer A to measure the spectrum.
Time-dependent extinction curves
Go to virtual lab course (Part 2, time-dependent measurements)Measure the extinction at the wavelength where the extinction was at a maximum for each of the four buffer solutions at 25 °C for a time span of four minutes each.
-
Fill the cuvette with a volume of 2.5 mL of buffer solution A and insert the cuvette into the photometer.
-
Measure the temperature of the filled cuvette. Wait until the temperature is constant at 25 °C for at least three minutes.
-
Open the software Extinktions-Zeit-Kurven.
-
Press Setup and enter the wavelength of the extinction maximum of brilliant green and confirm with Ok.
-
Press Start and enter the filename for your data as in the step above.
-
Add a volume of 0.3 mL of brilliant green solution with the micro pipette to the buffer solution. Mix the solution using the micro pipette.
-
To start the measurement press Ok in the window Loading Guide. Next, in the dialog window Sync Start press Ok.
-
Follow the measurement on the screen.
-
Remove the cuvette from the sample holder and check for impurities and air bubbles. Empty the cuvette into the waste container, clean the cuvette with pure water and dry the cuvette with lintfree cloth.
Repeat the measurement with buffer solutions B, C, and D.
Spectra at different time offset
Go to virtual lab course (Part 1, recording spectra)Record five spectra in the wavelength range of 250 nm to 800 nm at 25 °C. Each measurement should be offset from the other by three minutes. Use buffer solution D.
-
Fill the cuvette with a volume of 2.5 mL of buffer solution D and insert the cuvette into the photometer.
-
Measure the temperature of the filled cuvette. Wait until the temperature is constant for at least three minutes at 25 °C. For this, the thermometer probe can be inserted carefully in the cuvette.
-
Open the program zeitabhängige Spektren. The software indicates that no baseline correction is present. Confirm both alerts with Ok.
-
Create a baseline correction by pressing Baseline. The process is finished if the program is responsive again.
-
Press Start and choose a filename for your measurement (numbers and letters only). Choose the folder D:\Userdaten\ and create a sub directory with your group number. Confirm with Save.
-
Add 0.3 mL of brilliant green solution using the micro pipette to the water in the cuvette. Carefully mix the solution with the micro pipette (by inserting and releasing liquid into the micro pipette).
-
In the field Sample Name press Ok to start the measurement (the name given here does not play a role).
-
The software now records spectra during a time of twelve minutes (spaced three minutes apart). Follow the measurement on the monitor.
-
When the measurement is finished, press Finish in the window Sample Name.
-
Remove the cuvette from the sample holder and check for impurities and air bubbles. Empty the cuvette into the waste container, clean the cuvette with pure water and dry the cuvette with lintfree cloth.
-
When closing the program the values are saved automatically.
End of experiment
-
Discard all buffer solutions into the brilliant green waste container.
-
Copy the CSV files of your measurements onto your USB drive. The original files are kept on the computers and are not deleted.
-
Switch off the pH meter, the computer, the thermostat and the cooling circulation.
Analysis
Note: State all measured raw data values (including spectra) in your report.
-
Plot the extinction E as function of wavelength \lambda of brilliant green. Determine the wavelength \lambda_\text{max} where the extinction is at a maximum. Determine the error/uncertainty of \lambda_\text{max} considering the precision of your measurement.
-
Plot according to equation the expression \ln \left(\frac{E}{E_0}\right) against the time (in min) in a single diagram. Consider the significant places of the extinction values. Determine the reaction speed constant k and the corresponding error/uncertainty from the slope of a linear fit. Choose a reasonable value range for the linear fit. Discuss the influence of pH on the reaction speed constant k.
-
Plot the extinction E as function of wavelength \lambda for the spectra taken at different times in a single diagram. Discuss your data and state the wavelengths of the isosbestic point(s) including their error/uncertainty.
-
Plot according to equation the total reaction speed constant k as function of the hydroxide ion concentration c_\ce{OH^{-}}. Determine from a linear fit the reaction speed constants k'_1 and k'_2 as well as their error/uncertainty. Compare the reaction speed of the various reactions at different pH. Check whether you can reproduce the following previously measured values for the reaction speed constant k'_1 :
\begin{aligned} \text{pH} = \text{10.3} \pm \text{0.1} :\quad k'_1~&= \pu{(0.0025+-0.0005) / min}\\ \text{pH} = \text{10.6} \pm \text{0.1} :\quad k'_1~&= \pu{(0.0050+-0.0005) / min}\\ \text{pH} = \text{11.0} \pm \text{0.1} :\quad k'_1~&= \pu{(0.015+-0.005) / min}\\ \text{pH} = \text{11.6} \pm \text{0.1} :\quad k'_1~&= \pu{(0.049+-0.005) / min} \end{aligned}